July 3, 2023
Insights into the recycling of our cells
FIAS researchers have made a significant breakthrough in understanding the process of autophagy, a vital cellular mechanism responsible for the degradation and recycling of damaged cellular components. Their findings, obtained in collaboration with leading experts at the Francis Crick Institute in London and the University of Tokyo, were published in Science Advances and shed light on the role of a specific protein, ATG3, in this process.
Biological cells developed a sophisticated “housekeeping” system called autophagy. Cells are made of numerous smaller components, ranging from small molecules, sugars, and individual proteins to lipids that assemble in large membranes. In a cell, all these components are organized in an enormously complex choreography, necessary for the correct cellular functioning and, ultimately, the health and survival of the whole organism. Sometimes elements of this choreography get damaged—say, a protein complex or an organellar membrane, and the cell must eliminate them before they impede its normal functioning. In other situations, the cell can experience a lack of nutrients, such that it must recycle existing molecular complexes to focus on essential activities. In these situations, autophagy gets activated. From the Greek “self-eating,” autophagy is a complex pathway that involves the concerted action of many regulatory proteins that control the formation of a large vesicle, which will contain the cellular content meant for recycling. The protein ATG3 plays a crucial role in forming autophagosomes, the structures that encapsulate the materials to be degraded.
The research team around FIAS fellow Roberto Covino focused on a specific part of the ATG3 protein, known as the amphipathic α helix (AHATG3). They found that this component has unique biophysical properties that allow it to interact with membranes in a strictly controlled manner and are essential for the protein's function in autophagy.
Using advanced computational techniques integrated with cell biology experiments, the researchers could observe the dynamics of the ATG3 protein and its interaction with membranes during the autophagy process. They found that the unique properties of ATG3 are crucial for a key step in autophagosome formation.
These findings provide a deeper understanding of the molecular mechanisms underlying autophagy, which could have significant implications for developing treatments for diseases associated with impaired autophagy, such as neurodegenerative disorders and cancer.
Publication:
Taki Nishimura, Gianmarco Lazzeri, Noburu Mizushima, Roberto Covino, Sharon A. Tooze, Unique amphipathic α helix drives membrane insertion and enzymatic activity of ATG3, Science Advances 9:25, 2023, DOI: 10.1126/sciadv.adh1281
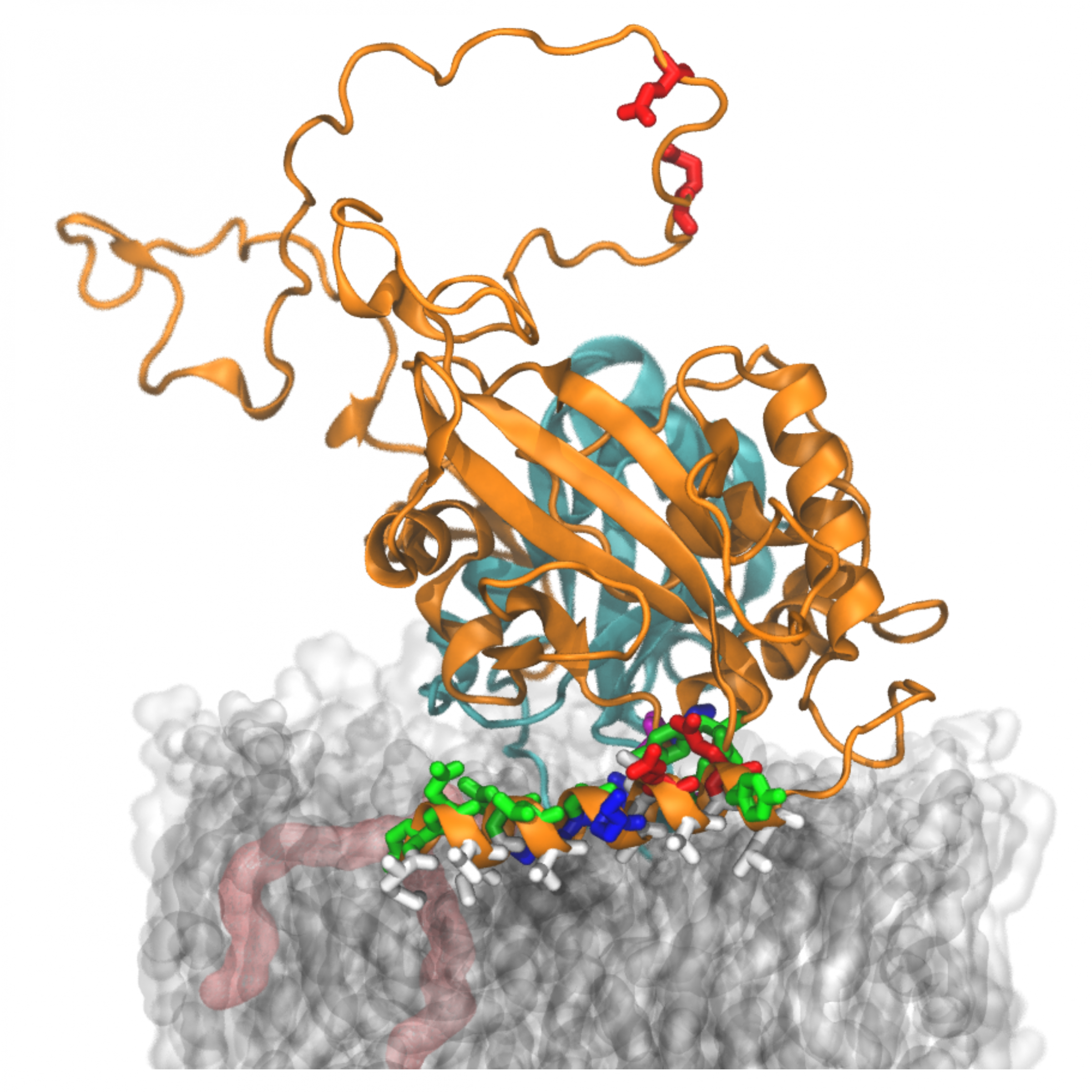
Figure:
Computer simulation of the protein complex ATG3 (orange) on a lipid membrane (grey/transparent). The researchers identified the part lying on the membrane (an amphipathic helix) as crucial for the process of autophagy. This basic recycling process in our cells is disturbed in certain diseases. (Gianmarco Lazzeri & Roberto Covino/FIAS)