September 7, 2022
New binding site to cell membrane identified
Role in retinal and hearing disorders
A team led by Sebastian Thallmair at FIAS investigates the interactions of proteins and lipids (fats) in membranes by means of simulations. In the barrel-shaped Tubby protein, they identified a previously unknown binding site that could help to understand various diseases.
If the Tubby protein does not work, disturbances in the energy balance, obesity, degeneration of the retina, and hearing loss are observed. The exact background for these malfunctions is unclear, therefore insights in the functioning of Tubby are important. The Tubby protein family plays an important role in the transport of proteins within the cell membrane. They bind receptor proteins in a precisely fitting "pocket" and channel them into fine hairs, the primary cilia. These cilia act as antennae that recognise signals outside the cell and transmit them into the cell interior. If their function and thus signal transmission is disturbed, diseases can occur (ciliopathies).
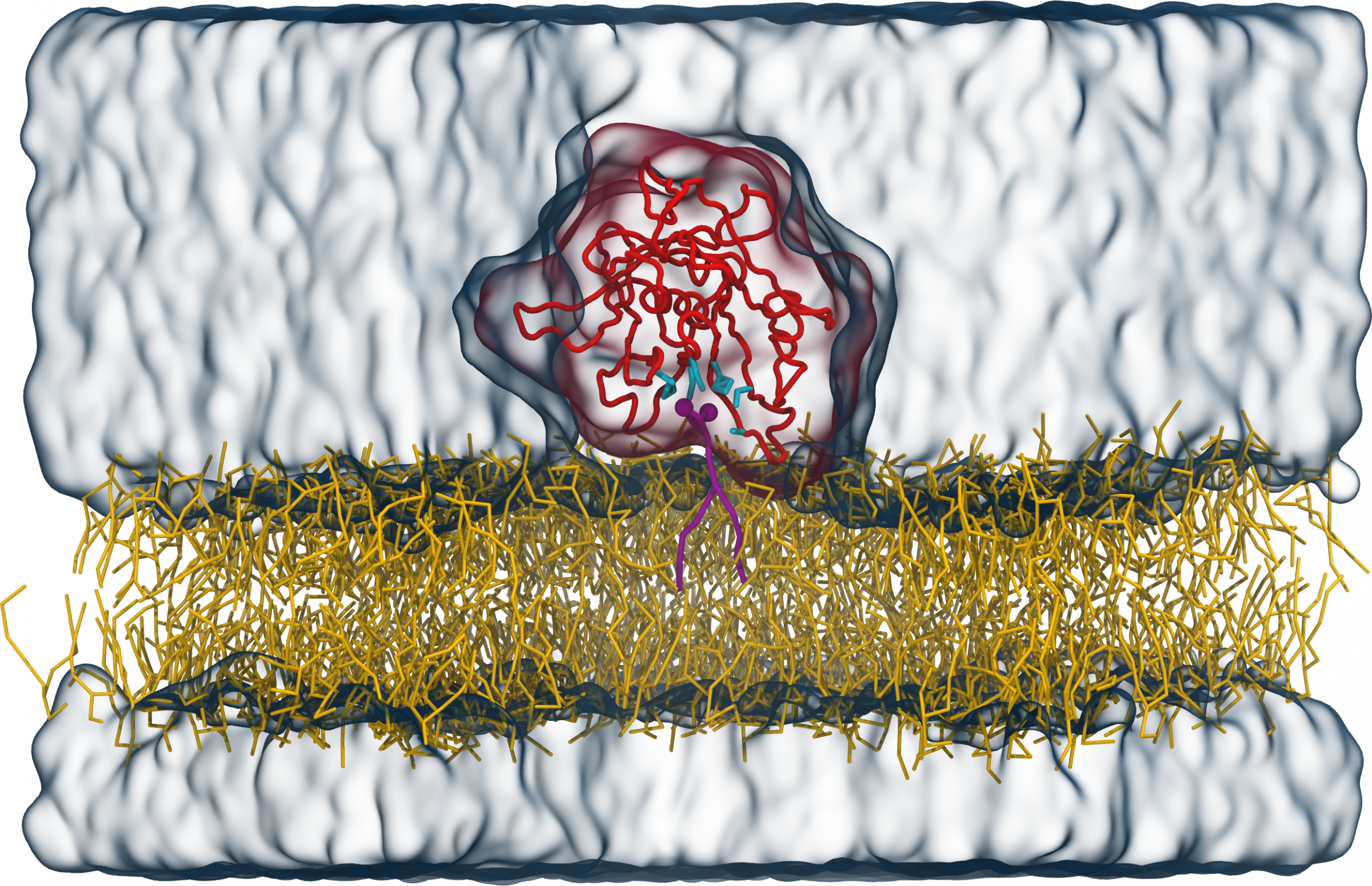
For Tubby to function, it must dock to the inside of the membrane via a specific lipid (PI(4,5)P2) that is found exclusively in the cell membrane. A team led by FIAS Fellow Dr. Sebastian Thallmair, Prof. Dr. Dominik Oliver (University of Marburg), and Prof. Dr. Siewert-Jan Marrink (University of Groningen, The Netherlands) investigated the binding mechanism of Tubby to this signalling lipid in more detail. In their current publication, they describe a previously unknown binding site of the Tubby protein. "Using computational modelling, we identified a second binding pocket for the signalling lipid, in addition to the already known one," says Thallmair. The basis for these model calculations are the known protein structure as well as information on chemical and physical binding preferences or repulsion reactions of individual atom groups.
To validate the modelling results, the team placed mutations in the predicted binding site - which subsequently does not function properly anymore. This confirmed that the second binding pocket is essential for Tubby's function in living cells. It plays a key role in enabling Tubby to dock to the cell membrane. Furthermore, the team showed that both binding sites cooperate. "This means that, surprisingly, two bound signalling lipids act more than twice as strongly as just one bound signalling lipid," explains Thallmair.
Fluorescently labelled Tubby protein is also used as a marker to draw conclusions about the lipid concentration of different membrane areas. "For instance, we would like to understand how the signalling lipid is re-synthesised after it has been degraded," says Thallmair. "It is obviously only synthesised in certain, narrowly defined areas of the cell membrane." Tubby should help to identify these areas.
Publication:
Veronika Thallmair, Lea Schultz, Wencai Zhao, Siewert Jan Marrink, Dominik Oliver, Sebastian Thallmair, Two cooperative binding sites sensitize PI(4,5)P2 recognition by the tubby domain, Sci. Adv. 8, eabp9471 (2022). DOI: 10.1126/sciadv.abp9471
Figure:
Tubby protein (red) on top of a lipid membrane (yellow) with a PI(4,5)P2 signalling lipid (purple) in the known binding pocket. The amino acids of the binding pocket a displayed in cyan, water as transparent blue surface. Figure reproduced from V. Thallmair et al., Sci. Adv. 8, eabp9471 (2022).