July 13, 2023
Sensor enables analysis of an important cellular scavenger
Glutathione concentration becomes measurable
During the metabolism of cells, aggressive oxygen compounds are formed as side products that can damage proteins or DNA. To intercept these oxygen compounds, cells use the peptide glutathione. A team of researchers from FIAS and the University of Zurich developed a novel sensor to measure the glutathione concentration in cell organelles. The sensor allows precise observation of the important molecule in cell organelles and supports research into related diseases.
Glutathione is a small peptide consisting of three amino acids. It plays a key role in the detoxification of aggressive oxygen compounds and is also involved in signal transmission in cells. It is present in the cells in a finely tuned balance of free glutathione and its dimeric form. Glutathione imbalances in the cell occur for instance cancer, diabetes and neurodegenerative diseases.
In order to better understand the glutathione balance and the associated disease triggers, methods are required to determine the amount of glutathione in cell organelles. So far, fluorescent proteins or small molecules are used as sensors that react with the glutathione molecules. However, the measurement in selected cell organelles has various weaknesses – e. g. the sensor has to cross the cell fluid and can already react with glutathione there.
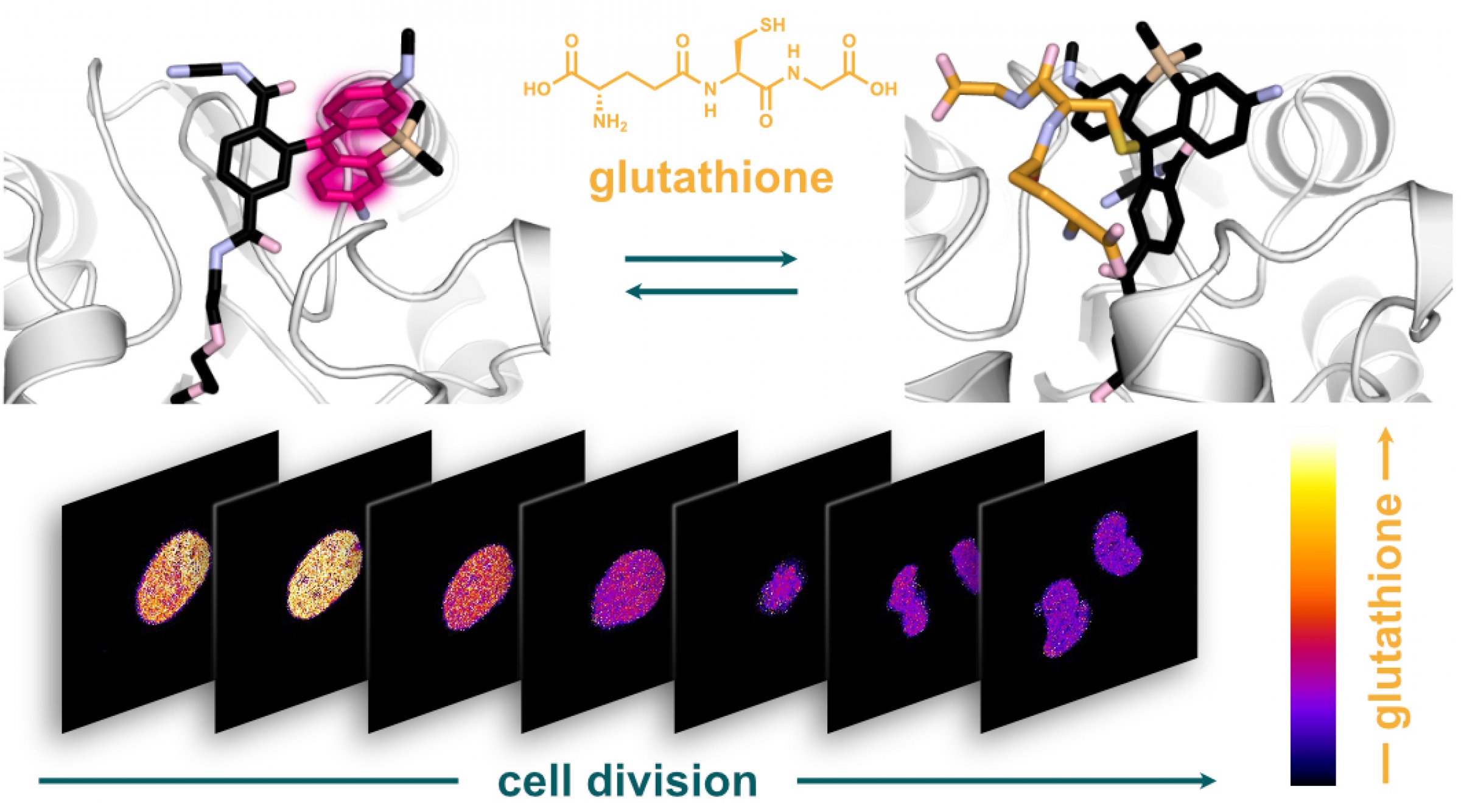
A team of researchers from the University of Zurich (Switzerland) and FIAS led by Sebastian Thallmair has now developed a glutathione sensor that is activated in cell organelles and thus allows reliable measurements of the glutathione concentration within cell organelles. The researchers used a combination of protein and silicon rhodamine (SiR) dye. The fluorescence of the dye changes from "off" to "on" as soon as it binds to the HaloTag protein. Since HaloTag can be steered in a desired cell organelle before reacting with the SiR dye, the sensor can be targeted in individual cell organelles.
The resulting hybrid sensor combines the advantages of genetically encoded sensors - such as targeting precision - with the tunability, brightness and photostability of small molecules. Unlike previous methods, the new sensor termed "targetable ratiometric quantitative glutathione" (TraQ-G) sensor only reacts in the organelle of interest.
TraQ-G can also be linked to a redox-insensitive fluorescent protein. This allows quantitative measurements of glutathione concentrations in living cells. The researchers were thus able to show that during cell division, the glutathione concentration in the cell nucleus and the cellular fluid are regulated independently.
The Thallmair group at FIAS simulated the combination of the HaloTag protein with different SiR dyes to understand their flexibility and reactivity with glutathione in more detail. The experimental work was carried out by Pablo Rivera-Fuentes' group at the University of Zurich. "Together, we aim to further improve the behaviour of the sensor,” Sebastian Thallmair describes the team’s future plans. They also want to extend the concept to develop improved fluorescence sensors for other central molecules in the cellular metabolism.
Figure caption: The HaloTag bound, fluorescent silicon rhodamine dye looses its fluorescence when reacting with glutathione (top). Using the novel sensor the researchers showed that the glutathione concentration in the cell nucleus varies strongly during cell division (bottom).
Publication:
Sarah Emmert, Gianluca Quargnali, Sebastian Thallmair und Pablo Rivera-Fuentes, A locally activatable sensor for robust quantification of organellar glutathione, Nature Chemistry (2023). https://doi.org/10.1038/s41557-023-01249-3